Here is the definition of specific heat: the amount of heat necessary for 1.00 gram of a substance to change 1.00 °C Note the two important factors:
Aug 26, 2007 · This is a simple experiment to measure the “specific heat” (also called “specific heat capacity”) of any fluid. In addition to the principle of
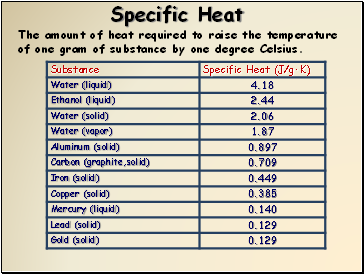
Consider the specific heat of copper , 0.385 J/g 0C. What this means is that it takes 0.385 Joules of heat to raise 1 gram of copper 1 degree celcius.
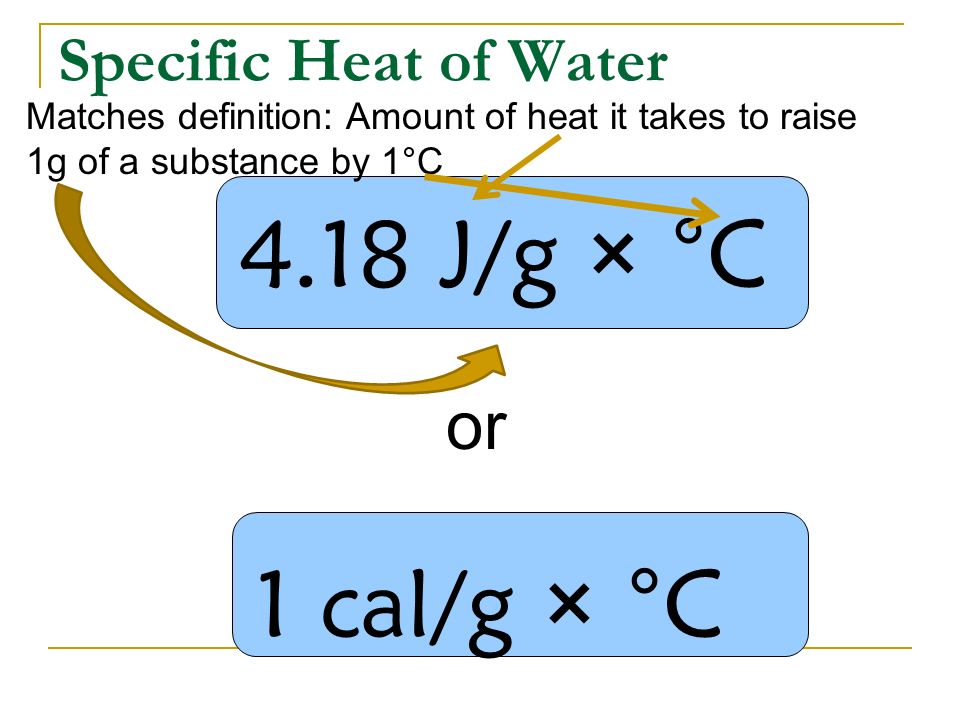

The specific heat is the amount of heat per unit mass required to raise the temperature by one degree Celsius. The relationship between heat and temperature change is usually expressed in the form shown below where c is the specific heat. The relationship does not apply if a phase change is
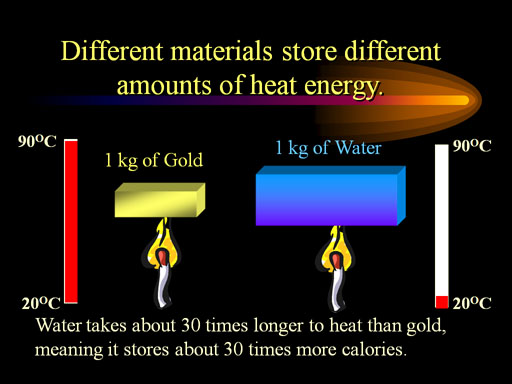
Specific heat: Specific heat, ratio of the quantity of heat required to raise the temperature of a body one degree to that required to raise the temperature of an equal mass of water one degree.
Example #1: We are going to determine the specific heat of copper metal. Now this has already been done many times, so the value is available in reference books. We will pretend that is not the case.


Online calculator, figures and tables showing specific heat of liquid water at constant volume or constant pressure at temperatures from 0 to 360 °C (32-700 °F) – …
SPECIFIC HEAT Reminder – Goggles must be worn at all times in the lab PRE-LAB DISCUSSION: The amount of heat required to raise the temperature of a solid body depends on its change in temperature

Measuring the heat capacity, sometimes referred to as specific heat, at constant volume can be prohibitively difficult for liquids and solids.
Specific heat capacity and heat of vaporization of water. Evaporative cooling. Why ice floats.
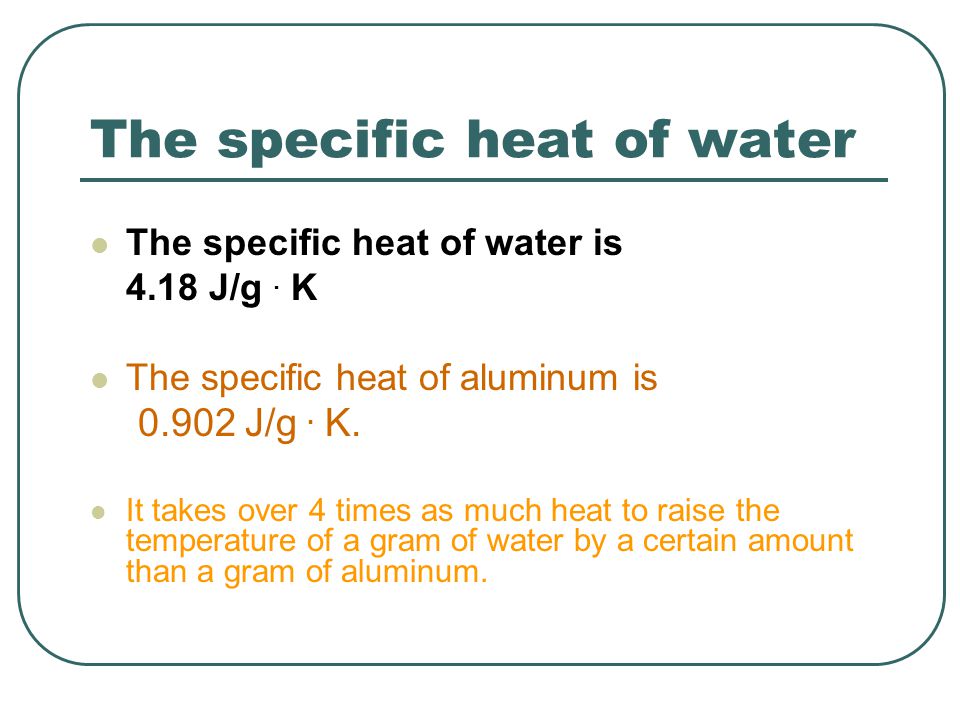
.PNG)
Recent Comments